The Glass Transition Temperature of the Acrylate Family
Introduction
The homopolymer and copolymers of 2-ethylhexyl acrylate (EHA) have very expert film formation characteristics and have very good low temperature flexibility, considering of the presence of branched and longer alkyl pendant grouping in the EHA. They have as well low volume shrinkage. So the copolymers of EHA are widely used in paints, coating and adhesive applications (Skeist, 1977; Plessis et al., 2001; Webster and Crain, 2002). Copolymerization is an important synthetic tool which can control the thermal and mechanical properties of the polymers (Kavousian et al., 2004). In the copolymers of styrene and EHA, the backdrop of the copolymers can be monitored by controlled incorporation of the respective comonomers styrene and EHA. Polystyrene possess high drinking glass transition temperature, T g (~100°C) where equally poly(2-ethylhexyl acrylate) (PEHA) has low T k of −threescore°C. Copolymers of styrene and EHA are of import components for difficult coating (Plessis et al., 2001) and also are used as blend compatibilizers (Haldankar, 2001). Conventional gratis radical polymerization (FRP) leads to uncontrolled molecular weight and broad dispersity ( Đ ). There is also gel germination tendency, because of the several side reactions during FRP. This makes them hard to apply for paints and coating textile application owing to high viscosity (Solomon and Moad, 1995; Odian, 2004). Since 1990s in that location take been spectacular advances in the field of controlled radical polymerization (CRP). There were several CRP techniques namely, atom transfer radical polymerization (ATRP) (Kamigaito et al., 2001; Matyjaszewski and Jia, 2001; Kavitha and Singha, 2008), nitroxide-mediated polymerization (NMP) (Harth et al., 2001; Hawker et al., 2001), and reversible addition–fragmentation concatenation transfer (RAFT) (Chiefari et al., 1998; Moad et al., 2000; Moad, 2006; Barner-Kowollik, 2008). Amidst the different CRP techniques, ATRP is applicative to polymerize wide range of monomers and can be carried out at wide range of temperature (−20 to 200°C) (Haloi et al., 2009). ATRP has been successfully applied to synthesize a wide range of polymers with varied molecular weights, unlike architectures, functionalities etc. Transition element catalyzed CRP known as ATRP is an important CRP method (Matyjaszewski, 2000) which is carried out in presence of an active alkyl halide using a transition metal halide as goad in combination with a suitable ligand (Matyjaszewski and Jia, 2001). A wide variety of copolymers can exist prepared via ATRP with controlled molecular weight, functionality, and low dispersity. At that place are several reports on the copolymerization of unlike acrylate monomers using ATRP and conclusion of their reactivity ratios. The reactivity ratios are important parameters for a set up of monomer. The reactivity ratios of monomers predict the copolymer composition every bit well as the sequence distribution of the comonomers. It also predicts the backdrop of the copolymer. ATRP provides random copolymer with similar chain compositions which is very much different from FRP (Matyjaszewski, 2002). During the polymerization reaction, the polymer chains grow simultaneously and thus all the polymer bondage accept same composition. However, in FRP macromolecular chains kickoff growing at different times during the polymerization and the monomer limerick continuously changes. As a result, in FRP the different bondage will have different compositions in the finish (Solomon and Moad, 1995). The reactivity ratios of co-monomers in a CRP are somewhat different from the same in FRP. This is because in CRP processes there is intermittent activation-deactivation of the agile species which results in different rates of consumption of comonomers (Matyjaszewski, 2002; Braunecker and Matyjaszewski, 2007). For example, Mignard et al. reported the reactivity ratios of the copolymerization of styrene and butyl acrylate (BA) via NMP at 120°C in solution. They reported the reactivity ratios for styrene and BA inside the range of 0.60–1.2 (rstyrene) and 0.16–0.29 (rBA) respectively (Mignard et al., 2004). Arehart and Matyjaszewski reported the reactivity ratios of styrene and BA prepared via ATRP at 110°C in solution. They reported the reactivity ratios for styrene and BA as 0.68 < rstyrene < 0.82 and 0.22 < rBA < 0.26 respectively (Arehart and Matyjaszewski, 1999). Chambard et al. reported the copolymerization of styrene and BA prepared in bulk via FRP. The reactivity ratios of styrene and BA prepared via FRP at ninety°C were reported to exist 0.95 and 0.20 respectively (Chambard et al., 1999). Ziegler and Matyjaszewski reported the variation in reactivity ratios of MMA and BA with the change in ligand from iv,4′-di(five-nonyl)-two,2′-bipyridine (dNbpy) (rMMA = 2.52, rBA = 0.26) to N,N,N′,N″,North″-pentamethyldiethylenetriamine (PMDETA) (rMMA = iii.15, and rBA = 0.37) (Ziegler and Matyjaszewski, 2001). Lessard et al. reported the reactivity ratios of styrene and tert-butyl acrylate(t-BA) prepared via NMP at 115°C in bulk equally r t-BA = 0.09–0.12 and rStyrene = 0.40–0.49 (Lessard et al., 2007). Nosotros reported the ATRP of furfuryl methylacrylate and methyl methacrylate (Kavitha and Singha, 2007), 2-ethylhexyl acrylate and glycidyl methacrylate (Haloi et al., 2009). Yet, there is no written report on the ATRP of styrene and EHA and to determine their reactivity ratios. The objective of this investigation is to study the copolymerization of styrene and EHA via ATRP and to make up one's mind their reactivity ratios. The reactivity ratios of styrene and EHA were calculated using Finemann–Ross (FR), inverted Finemann–Ross (IFR), and Kelen–Tudos (KT) methods (Fineman and Ross, 1950; Kelen et al., 1980; Makrikosta et al., 2005).
Materials and Methods
The monomers, 2-ethylhexyl acrylate (EHA) (Aldrich, Usa) and styrene (Jyoti Chemicals, Bombay) were purified by vacuum distillation. CuBr (Aldrich, USA) was purified by washing with glacial acetic acid, and then it was washed thoroughly with diethyl ether and was finally stale under vacuum. Phenyl ethylbromide (PEBr) (97%) and N,N,Due north′,N″,N″-pentamethyldiethylenetriamine (PMDETA) (97%) were purchased from Aldrich, United states and were used every bit received.
Characterization
Number boilerplate molecular weight (Mn, GPC) and dispersity ( Đ ) were determined by Gel Permeation Chromatography (GPC). GPC analysis was carried out at room temperature using a Viscotek GPC equipped with a refractive index detector (Model VE3580), 2 ViscoGEL GPC columns (model GMHHR-Thou # 17392) connected in serial. GPC assay was carried out using tetrahydrofuran every bit eluent at a flow rate of 1 ml/min. Linear and narrow disperse polystyrene was used as scale standard and Viscotek OMNI-01 software was used for information processing.
1H NMR spectra of the polymers were recorded on a 200 MHz Brucker NMR spectrometer using CDClthree every bit solvent which had a pocket-size amount of tetramethylsilane (TMS) as an internal standard.
Differential scanning calorimetry (DSC) analysis was carried out by using TA Instrument (DSC Q100 V8.one Build 251) nether nitrogen temper at a heating rate of 10°C/min inside a temperature range of −100°C to +150°C. The baseline calibration was done past scanning the temperature domain with the help of an empty pan. The enthalpy was calibrated past using indium standard and the oestrus capacity was calibrated by using the sapphire disc that was supplied by TA instrument. The drinking glass transition temperature (T g) was determined from the plot of heat menstruum vs. temperature in the second heating scan in the DSC assay.
Thermogravimetric assay (TGA) was carried out past using a TA Instrument (Q50) at a heating charge per unit of twenty°C/min in the temperature range of 30–600°C in nitrogen atmosphere. TGA analyzer consists of loftier precision balance with a pan which was placed in a small electrically heated oven. The temperature was measured accurately with the assistance of a thermocouple. From the plot of weight percent vs. temperature the polymer degradation temperature was determined.
Synthesis of Copolymers of Styrene and EHA via Atom Transfer Radical Copolymerization (ATRcP)
The polymerization reaction was carried out in a Schlenk tube. In a typical ATRP reaction EHA (4.82 g, 26.one mmol), styrene (0.909 g, 8.7 mmol) and CuBr (0.050 thousand, 0.35 mmol) were accurately weighed and transferred to the Schlenk tube. The PMDETA ligand (0.0604 1000, 0.35 mmol) was and so added to the reaction tube. Oxygen was removed from the reaction mixture by passing nitrogen through the reaction tube. The polymerization was started by adding PEBr (0.0646 g, 0.35 mmol) and was carried out at 90°C. Aliquot samples were taken out at different time intervals and were used to summate the conversion by gravimetric method. The samples were also used to find out the molecular weight past GPC. The last product was diluted with THF and was purified by passing through alumina cavalcade to remove the copper catalyst. The same procedure was adopted for other feed ratios of ATRcP (atom transfer radical copolymerization) of EHA and styrene.
Homopolymerization of Styrene via Atom Transfer Radical Polymerization (ATRP)
The homopolymerization of styrene was carried out in bulk in a Schlenk tube equipped with silicone septum and magnetic stirring bar. In the Schlenk tube styrene (4.5 one thousand, 43.2 mmol), CuBr (0.031 g, 0.21 mmol) and PMDETA (0.037 chiliad, 0.21 mmol) were weighed and degassed past passing nitrogen gas for 15 min. The reaction was started by adding PEBr (0.040 yard, 0.21 mmol) in the mixture. The reaction was carried out at 110°C for 6 h. oneH NMR (CDCl3, 200 MHz): δ (in ppm) = half dozen.4–7.2 (phenyl protons of polystyrene) and 1.four–2.ii ppm (−CH2− and >CH− protons).
Synthesis of Diblock Copolymer of Polystyrene with EHA via ATRP
The homopolymer of polystyrene (i.e., PS-Br) was used equally macroinitiator for the synthesis of diblock copolymer of styrene and EHA. The macroinitiator, PS-Br (1.0 yard, 0.066 mmol) was taken in a Schlenk tube and was dissolved in THF solvent into which CuBr (0.009 g, 0.069 mmol) and PMDETA (0.017 g, 0.10 mmol) were added followed by EHA (1 thousand, v.4 mmol). The polymerization reaction was carried out at 90°C for vi h. The polymer obtained was dissolved in THF and was purified by passing through basic alumina column and then precipitated in methanol.
Results and Word
Copolymerization of styrene and 2-ethylhexyl acrylate was carried out via ATRcP (shown in Scheme ane) at different feed ratios past using phenylethylbromide (PEBr) as initiator, CuBr as catalyst in combination with PMDETA as a ligand. From the kinetic plot of ln(one/1-Ten) (where X is the percent conversion of monomer) vs. time, information technology was observed that the value of ln(1/1-Ten) was linearly increased with fourth dimension (Effigy one). This linear dependency is the characteristics of the controlled polymerization reaction which follows the beginning order kinetics. Figure 1 showed that with increase in styrene content in the feed the rate of polymerization increased (Jianying et al., 2006). Effigy two infers that there was linear increase in molecular weight with conversion and dispersity ( Đ ) was relatively narrow. GPC traces of poly(styrene-co-EHA) (50:l) (sample 3 of Table one) is shown in supplementary department (Figure S1). It indicates the controlled nature of the copolymerization reaction. Table 1 summarizes the feed ratio too as the copolymer composition of the different copolymerization reactions.

Scheme 1. Copolymerization of styrene and EHA via cantlet transfer radical polymerization (ATRP).
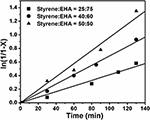
Figure 1. Kinetic plot of ln[ane/(1-X)] vs fourth dimension for copolymerization of styrene and EHA. [PEBr]: [M]o:[CuBr]:[PMDETA] = 1:100:one:i, at ninety°C.
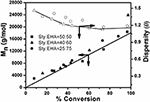
Figure 2. Plot of M n and Đ vs. conversion (%) for ATRcP of Styrene and EHA in bulk. [PEBr]: [K]o:[CuBr]:[PMDETA] = 1:100:i:i, at 90°C.

Table 1. Copolymerization of styrene and EHA in bulk at 90°C.
Structural Characterization and Copolymer Composition
The structural characterization and copolymer limerick were determined by 1H NMR spectroscopy. Figure 3 shows the 1H NMR spectra of poly(styrene-co-EHA) of xl:threescore feed ratio. The resonance at δ = 0.eight ppm is due to the –CH3 protons of PEHA part. The wide resonances at δ = 1.0 to 2.1 ppm are due to the unlike –CHtwo– and >CH– protons of pendant group of PEHA part as well as those of the master chain backbone of the copolymer. The resonances at δ = six.five–7.i ppm are due to the different aromatic protons of polystyrene office. The resonances at iii.8 ppm are due to –OCH2– protons of PEHA office. The distinct resonances at 6.5–seven.3 for v aromatic protons of styrene and three.viii ppm for 2 protons of EHA were used to calculate the limerick of the copolymer of styrene and EHA. The copolymer composition was determined past the equation (1) every bit shown beneath
where, A and B stand for the integral area at δ = iii.7 and δ = half dozen.5–7.iii ppm for –OCH2– protons in PEHA and aromatic protons of polystyrene unit respectively.
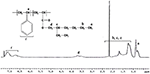
Effigy iii. 1H NMR spectrum of poly(styrene-co-EHA) with feed tooth limerick 50:50.
Reactivity Ratio Decision
In the copolymerization of styrene (one) and EHA (2) the reactivity ratio r1 is divers as k11/k12, where g11 is the rate constant of the reaction between the growing polymer chain conveying free radical of styrene as the last unit and styrene (homo propagation) and 100012 is the rate constant of the reaction betwixt the same reactive chain end and the EHA monomer. Similarly the reactivity ratio r2 is also defined equally thousand22/m21, where k22 is the rate abiding of homo propagation reaction between the growing macromolecular chain having EHA active radical equally the terminal unit and EHA and k21 is the rate abiding of the reaction between EHA active radical and styrene monomer (cross propagation). For determining the rone and rtwo, copolymerization of styrene and EHA was carried out at different feed ratio of styrene (i) and EHA (two). In this case copolymerization was carried out at low conversion (~x%) and its tooth composition was adamant by aneH NMR spectroscopy. Composition of the depression conversion copolymer was used for the determination of monomer reactivity ratios. In this case FR, IFR, and KT methods were used to decide the reactivity ratio of the monomers.
In the FR method the post-obit equation was used
where, M = molar feed ratio (M1/M2) and P = copolymer composition (grandane/ktwo)
where, Yard = M−M/P and H = Mii/P
The plot of G vs. H gives the straight line (Figure 4). From this the slope and intercept were calculated to be ri = 1.24 and rii = 0.71 respectively.
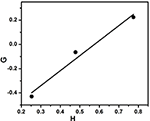
Figure iv. Finemann–Ross plot for copolymerization of styrene with EHA.
In the IFR method the equation used is
And then, from the plot of 1000/H and i/H (Effigy v) the reactivity ratios, r1 and rtwo were calculated as 1.34 and 0.76 from the intercept and the gradient respectively.
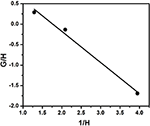
Figure 5. Inverted Finemann–Ross plot for copolymerization of styrene with EHA.
In KT method the reactivity ratio was determined by using the equation
where, η = G/(∝+H) and ξ = H/(∝+H) and ∝ = (Hmin. Hmax)1/ii, Hmin = 0.253 and Hmax = 0.775. Hmin and Hmax values are taken from Table ii.

Table 2. Finemann–Ross, Inverted Finemann–Ross and Kelen–Tudos parameter for the copolymer of styrene and EHA in majority*.
In the plot of η vs. ξ, (Figure 6) the slope gives the value of (r1 + rtwo/∝) and intercept provides r2/∝. From these two values r1 and rtwo were calculated equally rane = i.30 and rtwo = 0.73 respectively. All the parameters used for the three methods are given in Tabular array 2.
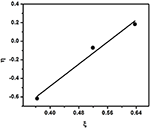
Figure 6. Kelen-Tudos plot for copolymerization of styrene with EHA.
The reactivity ratios, r1 and r2 calculated past the different methods are tabulated in Table iii. They are quite comparable. The values; r1 > ane, r2 < one and r2 < ri indicate that the styrene has much influence on the copolymer germination during the reaction (Jianying et al., 2006). Srivastava et al. (Srivastava and Rai, 1995) reported the copolymerization of styrene and EHA initiated by azobisisobutyronitrile (AIBN) in bulk in the presence of anhydrous ZnCl2. They reported the reactivity ratios for styrene and EHA equally 0.10 and 0.175 respectively. Moreover, Kavousian et al. reported the copolymerization of styrene and EHA via conventional radical polymerization (Kavousian et al., 2004). They reported the reactivity ratio of styrene and EHA as 0.926 and 0.238 respectively. At that place is a difference in the polymerization mechanism of ATRP and non-ATRP processes. In ATRP, atom transfer from an organic halide to a transition metallic circuitous occurs to generate the active radical species, which are then quickly "deactivated" by back transfer of the atom from the transition metal to the radical species (Matyjaszewski, 2002; Braunecker and Matyjaszewski, 2007). So, there is departure in reactivity ratio values in comparing to FRP. Barim et al. studied the FRP and ATRP of phenoxycarbonylmethyl methacrylate (PCMMA) and styrene at 110°C. They reported the reactivity ratios of PCMMA and styrene prepared via ATRP were 0.33 and 0.96 respectively and the same prepared via FRP were 0.47 and one.16 respectively (Barim et al., 2007). The reactivity ratio varies with the polymerization temperature (Chambard et al., 1999; McManus et al., 2002). Nosotros did the polymerization reaction via ATRP in bulk at 90°C. Nonetheless, the reactivity ratios calculated in this work follow the aforementioned trend (rEHA < rstyrene) equally reported by the other authors. In addition, in ATRcP technique the production of the reactivity ratios is less than 1. Information technology shows the trend of random copolymer formation, where the chances of incorporation of styrene is more in comparison to EHA.

Tabular array 3. Reactivity ratio of styrene (r1) and EHA (r2) adamant by three different models.
The mean sequence length (fifty) of the copolymers were determined by using the equations (Pazhanisamy et al., 1997)
and
where, rane (styrene) = one.29 and r2 (EHA) = 0.73
The results of the mean sequence length in the copolymer are shown in Table 4. It indicates that the length of EHA increases every bit its content in the monomer feed increases.

Table 4. The monomer composition and sequence length ratio.
Thermal Properties
The glass transition temperature (T g) of the copolymer was determined by DSC assay (shown in Figure 7). All the copolymers showed a single T g and they are shown in Table 5. It indicates that as the EHA (T 1000 = −60°C) content increases the T g decreases. The T yard for the copolymers adamant past DSC assay was compared with the same (T g, F-F) determined by Flory-Fox equation (Lijia et al., 1997). There is consistent difference between the T g values at dissimilar content of two comonomers (Table 5). Table 5 shows that there is some discrepancy between the experimental T grand and T chiliad calculated by Flory-Play tricks equation. The Flory-Pull a fast one on model is based on the free book theory. The discrepancy in the T g values is due to the fact that the effect of the chemical nature and organization of the monomers on the mobility of a polymer concatenation was non considered (Fernandez-Garcia et al., 1999).
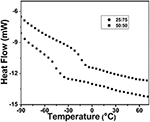
Figure vii. DSC thermogram of poly(styrene-co-EHA).

Tabular array 5. Thermal properties of copolymers of styrene and EHA.
The block copolymer of styrene and EHA (PS-b-PEHA) was prepared by using polystyrene-Br as macroinitiator. The shift of GPC traces of the diblock copolymer toward lower elution volume indicated the successful grooming of cake copolymer. (The GPC traces are shown in Figure S2). This block copolymer showed two T gdue south, PEHA block at −64°C and polystyrene at +123°C (Effigy 8). Withal, the copolymers, poly(styrene-co-EHA) showed only ane T g indicating the copolymers were not blocky in nature. Thermal stability of the copolymer was studied by TGA (Effigy 9). It is articulate that equally the styrene content increases, there is a slight increase in T onset. Even so, there was no significant change in T max every bit shown in Tabular array v.
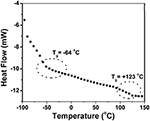
Figure 8. DSC thermogram of the polystyrene-b-PEHA.
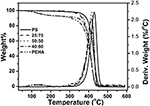
Effigy 9. TGA thermogram of homopolymer and copolymers of styrene and EHA.
Conclusions
Copolymers of styrene and 2-ethylhexyl acrylate were synthesized successfully in bulk by using atom transfer radical copolymerization (ATRcP). The chemical limerick was studied past 1H NMR spectroscopy and the reactivity ratios of the two monomers were calculated by using FR, IFR, and KT methods. The reactivity ratios of styrene and EHA were somewhat different from the polymerization reaction of styrene and EHA using the FRP organization. DSC analysis showed that the T g of the copolymer increases on increasing styrene content.
Conflict of Interest Statement
The authors declare that the inquiry was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
Bishnu P. Koiry gratefully acknowledges the fellowship from IIT, Kharagpur, Bharat.
Supplementary Material
The Supplementary Material for this article can exist plant online at: http://www.frontiersin.org/journal/ten.3389/fchem.2014.00091/abstract
Effigy S1. GPC traces of poly(styrene-co-EHA), sample 3 of Table ane.
Figure S2. GPC traces of polystyrene macroinitiator and polystyrene-b-PEHA cake copolymer.
References
Arehart, South. V., and Matyjaszewski, One thousand. (1999). Atom transfer radical copolymerization of styrene and n-butyl acrylate. Macromolecules 32, 2221–2231. doi: x.1021/ma981693v
CrossRef Full Text | Google Scholar
Barim, 1000., Demirelli, Chiliad., and Coskun, Grand. (2007). Conventional and atom transfer radical copolymerization of phenoxycarbonylmethyl methacrylate-styrene and thermal beliefs of their copolymers. Express Polym. Lett. 1, 535–544. doi: 10.3144/expresspolymlett.2007.76
CrossRef Full Text | Google Scholar
Barner-Kowollik, C. (2008). Handbook of RAFT Polymerization. Weinheim: Wiley-VCH Verlag GmbH and Co. KGaA. doi: 10.1002/9783527622757
CrossRef Full Text | Google Scholar
Braunecker, W. A., and Matyjaszewski, K. (2007). Controlled/living radical polymerization: features, developments, and perspectives. Prog. Polym. Sci. 32, 93–146. doi: x.1016/j.progpolymsci.2006.11.002
CrossRef Full Text | Google Scholar
Chambard, G., Klumperman, B., and German, A. 50. (1999). Dependence of chemical limerick of styrene/butyl acrylate copolymers on temperature and molecular weight. Polymer 40, 4459–4463. doi: 10.1016/S0032-3861(98)00690-9
CrossRef Total Text | Google Scholar
Chiefari, J., Chong, Y. K., Ercole, F., Krstina, J., Jeffery, J., Le, T. P. T., et al. (1998). Living costless-radical polymerization by reversible addition-fragmentation chain transfer: the RAFT process. Macromolecules 31, 5559–5562. doi: 10.1021/ma9804951
CrossRef Full Text | Google Scholar
Fernandez-Garcia, M., Cuervo–Rodriguez, R., and Madruga, East. L. (1999). Glass transition temperatures of butyl acrylate–methyl methacrylate copolymers. J. Polym. Sci. B Polym. Phys. 37, 2512–2520. doi: 10.1021/ma001182k
CrossRef Full Text | Google Scholar
Fineman, Thou., and Ross, S. D. (1950). Liner method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 5, 259–262. doi: 10.1002/pol.1950.120050210
CrossRef Full Text | Google Scholar
Haldankar, 1000. S. (2001). Compatibilizer for Polymer Blends. Frankfort, IL: The Sherwin-Williams Co. US Patent 6,541,571.
Google Scholar
Haloi, D. J., Roy, Due south., and Singha, North. Yard. (2009). Copper catalyzed cantlet transfer radical copolymerization of glycidyl methacrylate and two-ethylhexyl acrylate. J. Polym. Sci. A Polym. Chem. 47, 6526–6533. doi: 10.1002/pola.23695
CrossRef Full Text | Google Scholar
Harth, East., Horn, B. Five., and Hawker, C. J. (2001). Dispatch in nitroxide mediated 'living' free radical polymerizations. Chem. Commun. 9, 823–824. doi: x.1039/b102145c
CrossRef Full Text | Google Scholar
Jianying, H., Jiayan, C., Jiaming, Z., Yihong, C., Lizong, D., and Yousi, Z. (2006). Some monomer reactivity ratios of styrene and (meth)acrylates in the presence of TEMPO. J. Appl. Polym. Sci. 100, 3531–3535. doi: ten.1002/app.22512
CrossRef Total Text | Google Scholar
Kavitha, A., and Singha, Northward. K. (2007). Atom-transfer radical copolymerization of furfuryl methacrylate (FMA) and methyl methacrylate (MMA): a thermally-amendable copolymer. Macromol. Chem. Phys. 208, 2569–2577. doi: x.1002/macp.200700239
CrossRef Full Text | Google Scholar
Kavitha, A., and Singha, N. One thousand. (2008). High temperature resistant tailor-fabricated poly(meth)acrylates bearing adamantyl group via atom transfer radical polymerization. J. Polym. Sci. A Polym. Chem. 46, 7101–7113. doi: 10.1002/pola.23015
CrossRef Full Text | Google Scholar
Kavousian, A., Ziaee, F., Nekoomanesh, H., Leamen, G. J., and Penlidis, A. (2004). Determination of monomer reactivity ratios in styrene/2-ethylhexylacrylate copolymer. J. Appl. Polym. Sci. 92, 3368–3370. doi: 10.1002/app.20338
CrossRef Full Text | Google Scholar
Kelen, T., Tudos, F., and Turcsanyi, B. (1980). Confidence intervals for copolymerization reactivity ratios determined by the Kelen-Tudos method. Polym. Bull. 2, 71–76. doi: x.1007/BF00275556
CrossRef Full Text | Google Scholar
Lessard, B., Graffe, A., and Maric, M. (2007). Styrene/t-Butyl acrylate random copolymers synthesized by nitroxide-mediated polymerization: result of gratis nitroxide on kinetics and copolymer composition. Macromolecules forty, 9284–9292. doi: 10.1021/ma071689w
CrossRef Full Text | Google Scholar
Lijia, A. N., Dayong, H. E., Jlaokai, J., Zhigang, W., Donghong, Y. U., Jiang, B., et al. (1997). Effects of molecular weight and interaction parameter on the glass transition temperature of polystyrene mixture and its blends with polystyrene/poly(2,6-dimethyl-p-phenylene oxide). Eur. Polym. J. 33, 1523–1528. doi: 10.1016/S0014-3057(97)00089-X
CrossRef Total Text | Google Scholar
Makrikosta, G., Georgas, D., Siakali-Kioulafa, E., and Pitsikalis, Yard. (2005). Statistical copolymers of styrene and 2-vinylpyridine with trimethylsilyl methacrylate and trimethylsilyloxyethyl methacrylate. Eur. Polym. J. 41, 47–54. doi: 10.1016/j.eurpolymj.2004.08.001
CrossRef Full Text | Google Scholar
Matyjaszewski, K. (2000). "Controlled/Living radical polymerisation: progress in ATRP, NMP and RAFT," in ACS Symposium Series (New Orleans, LA). doi: 10.1021/bk-2000-0768
CrossRef Full Text | Google Scholar
Matyjaszewski, Chiliad. (2002). Factors affecting rates of comonomer consumption in copolymerization processes with intermittent activation. Macromolecules 35, 6773–6781. doi: 10.1021/ma0208180
CrossRef Full Text | Google Scholar
McManus, N. T., Penlidis, A., and Dube, Chiliad. A. (2002). Copolymerization of blastoff-methyl styrene with butyl acrylate in bulk. Polymer 43, 1607–1614. doi: ten.1016/S0032-3861(01)00738-8
CrossRef Full Text | Google Scholar
Mignard, East., Leblanc, T., Bertin, D., Guerret, O., and Reed, Westward. F. (2004). Online monitoring of controlled radical polymerization: nitroxide-mediated gradient copolymerization. Macromolecules 37, 966–975. doi: 10.1021/ma035589b
CrossRef Full Text | Google Scholar
Moad, Thousand., Chiefari, J., Chong, Y. K., Krstina, J., Mayadunne, R. T. A., Postma, A., et al. (2000). Living free radical polymerization with reversible improver–fragmentation chain transfer (the life of RAFT). Polym. Int. 49, 993–1001. doi: 10.1002/1097-0126(200009)49:nine<993::Aid-PI506>3.0.CO;2-half-dozen
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Odian, One thousand. (2004). Principles of Polymerization. New Jersey, NJ: Wiley-Interscience publication. doi: 10.1002/047147875X
CrossRef Full Text
Pazhanisamy, P., Ariff, Grand., and Anwaruddin, Q. (1997). Copolymers of α-methylstyrene with Northward-cyclohexylacrylamide: synthesis, monomer reactivity ratios, and hateful sequence length. J. Macromol. Sci. A Pure Appl. Chem. A34, 1045–1054. doi: x.1080/10601329708015009
CrossRef Full Text | Google Scholar
Plessis, C., Arzamendi, G., Alberdi, J. M., Agnely, Grand., Leiza, J. R., and Asua, J. M. (2001). Intramolecular chain transfer to polymer in the emulsion polymerization of ii-ethylhexyl acrylate. Macromolecules 34, 6138–6143. doi: ten.1021/ma0018190
CrossRef Full Text | Google Scholar
Skeist, I. (1977). Handbook of Adhesives. New York, NY: Chapman & Hall.
Google Scholar
Solomon, D. H., and Moad, M. (1995). The Chemical science of Free Radical Polymerization. Oxford: Elsevier Science Ltd.
Google Scholar
Srivastava, N., and Rai, J. Due south. P. (1995). Kinetics, mechanism, and rheological properties of the copolymers of ii-ethylhexylacrylate and styrene. J. Macromol. Sci. A Pure Appl. Chem. A 32, 2049–2062. doi: 10.1080/10601329508011044
CrossRef Full Text | Google Scholar
Webster, D. C., and Crain, A. L. (2002). Synthesis of latexes containing diesters of 3-butene-ane,2-diol. Prog. Org. Glaze. 45, 43–48. doi: 10.1016/S0300-9440(02)00099-1
CrossRef Full Text | Google Scholar
Ziegler, G. J., and Matyjaszewski, M. (2001). Atom transfer radical copolymerization of methyl, methacrylate and north-butyl acrylate. Macromolecules 34, 415–424. doi: ten.1021/ma001182k
CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fchem.2014.00091/full
0 Response to "The Glass Transition Temperature of the Acrylate Family"
Postar um comentário